Tell the FDA we need boosters authorized based on scientific evidence.
Comments close Wed. January 25th, 11:59PM - it is imperative we make our voices heard.
Dear Friends,
On Thursday January 26, the FDA decides whether boosters are authorized every 6 months or merely annually. It is imperative we make our voices heard.
Public Comments are being accepted through Wed. January 25th, 11:59PM:
https://www.regulations.gov/document/FDA-2022-N-2810-0001
(NOTE: All comments are shared and published publicly.)
(For category choose "Individual Consumer")
SAMPLE COMMENT
Docket No. FDA-2022-N-2810
Scientific evidence indicates the vaccine should ideally be allowed, available, and fully covered by public funds or insurance, twice a year.
The FDA’s decision will affect everything about the vaccine drive including what doctors recommend, what the public considers, and what HEALTH INSURANCE COVERS.
If it’s only recommended annually, that may create barriers for vulnerable people, and may discourage high risk people from getting needed boosters.
References:
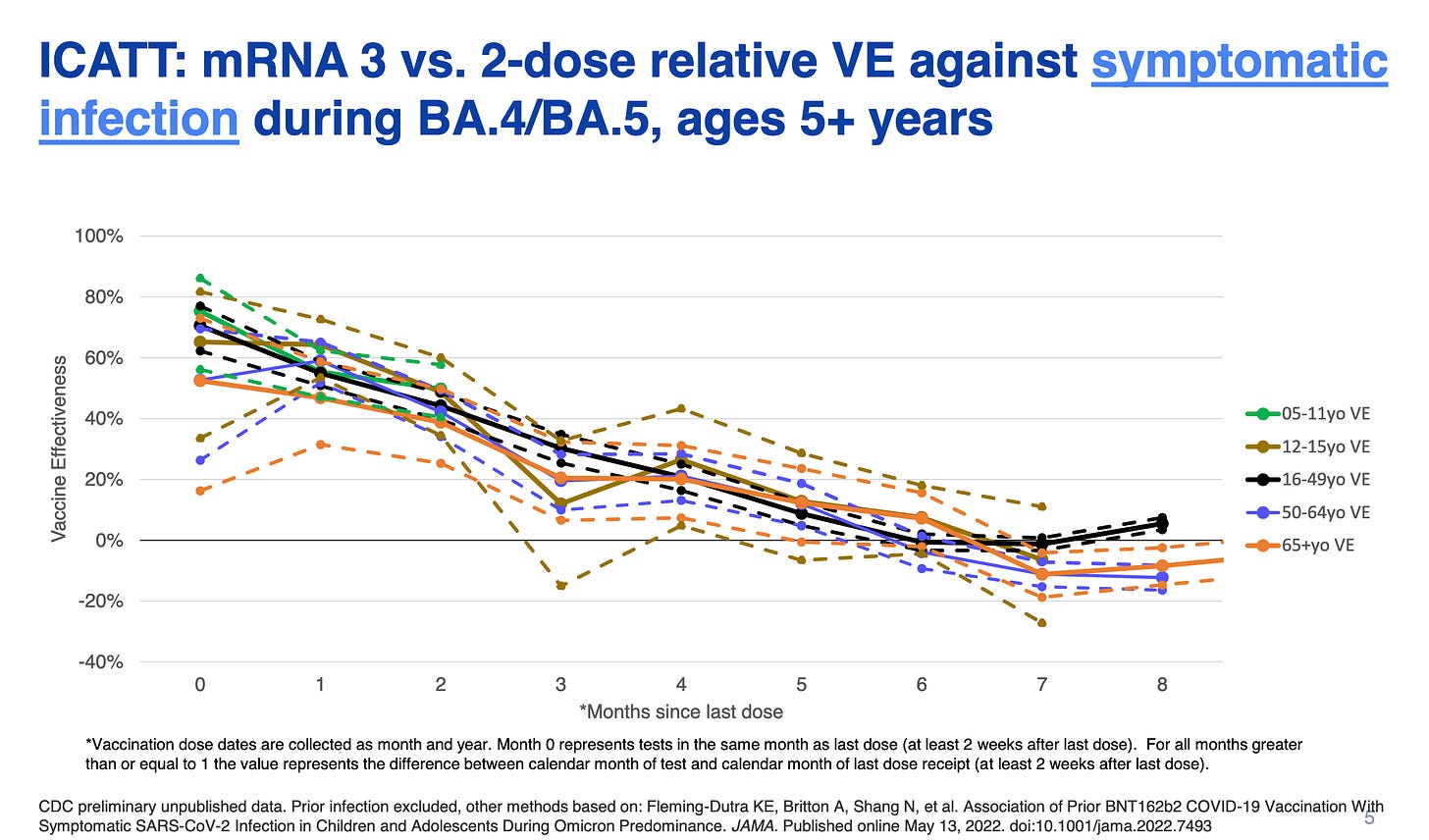
CDC: Advisory Committee on Immunization Practices (ACIP) Presentation Slides: September 1-2, 2022 Meeting - The presentation by Ruth Link-Gelles showed evidence that there is profound waning of the vaccines against any symptomatic infection by 6 months.
Our analysis strongly supports boosting on an annual or more frequent cycle to markedly diminish the long-term risk of infection.
Kristian Andersen, an immunologist at Scripps Research, has contended that people need to be boosted every six months or so. "We just need to realize that immunity, unfortunately, wanes pretty quickly," Andersen said. "We don't want that to be true. We want lifelong immunity. We want measles-type immunity." He said that is wishful thinking at the moment.
[Emphasis added]


